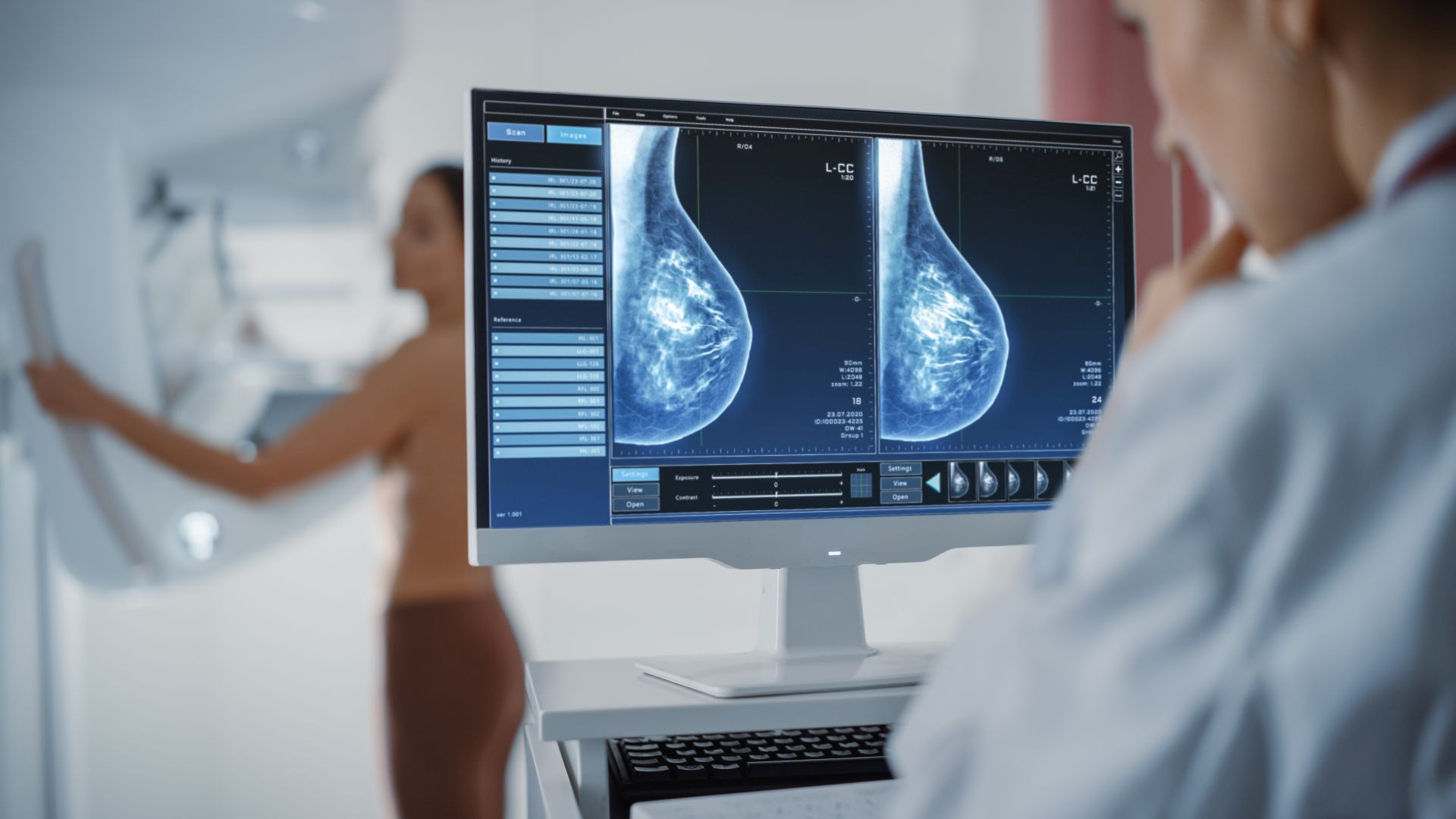
Spore.Bio has appointed Michael Miller as vp of scientific and [...]
Mental health systems in the US and UK have entered [...]
MaaT Pharma has reported its half year 2025 financial results, [...]
Donidalorsen has received European Union marketing authorisation for the routine [...]
The Parenteral Drug Association will host a half-day workshop focused [...]
Patients in Scotland living with extensive-stage small cell lung cancer [...]
Minovia Therapeutics has been granted two new US patents covering [...]
Merck has announced that the US Food and Drug Administration [...]
Cellares has secured FDA clearance of an IND amendment allowing [...]
Dan Beaudry, market owner for risk-based quality management (RBQM) at [...]